|
 |
|
 |
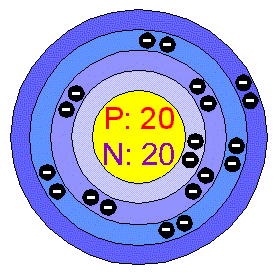
CalciumThe chemical element Calcium (Ca), atomic number 20, is the fifth element and the third most abundant metal in the earth’s crust. The metal is trimorphic, harder than Sodium, but softer than Aluminium. A well as Beryllium and Aluminium, and unlike the alkaline metals, it doesn’t cause skin-burns. It is less chemically reactive than alkaline metals and than the other alkaline-earth metals. Calcium ions solved in water form deposits in pipes and boilers and when the water is hard, that is, when it contains too much Calcium or Magnesium. This can be avoided with the water softeners. In the industry, metallic Calcium is separated from the melted Calcium Chloride by electrolysis. This is obtained by treatment of Carbonated minerals with chlorhydric acid, or like a sub product of the Carbonates Solvay process. In contact with air, Calcium develops an oxide and nitride coating, which protects it from further corrosion. It burns in the air at a high temperature to produce nitride. The commercially produced metal reacts easily with water and acids and it produces Hydrogen which contains remarkable amounts of ammonia and hydrocarbides as impurities. Applications The metal is used in Aluminium alloys for bearings, as a helper in the Bismuth removal form lead, as well as in controlling graphitic Carbon in melted Iron. It is also used as a deoxidizer in the manufacture of many steels; as a reducing agent in the preparation of metals as Chromium, Thorium, Zirconium and Uranium, and as separating material for gaseous mixtures of Nitrogen and Argon. Calcium is an alloying used in the production of Alluminium, Beryllium, Copper, lead and Magnesium alloys. It is also used in making cements and mortar that are used in builldings. The Calcium oxide, CaO, is produced by thermal decomposition of Carbonated minerals in furnaces, applying a continuous bed process. The oxide is used in high intensity light arcs (lime light) for its unusual spectral characteristics and as dehydrating industrial agent. The metallurgic industry extensively uses the oxide during the reduction of ferrous alloys. The Calcium oxide, Ca(OH)2, has many applications in which the hydroxyl ion is necessary. In the process of Calcium hydroxide quenching, the volume of blown out lime [Ca(OH)2] expends to double the initial quantity of quick lime (CaO), fact that makes it useful to break down rocks or wood. The quick lime is an excellent absorbent for the Carbon dioxide, because it produces Carbonate, which is very insoluble. The Calcium silicate, CaSi, prepared in an electric oven from lime, silica and reducing Carbonated agents, is useful as a steel-deoxidizing agent. Calcium carbide, CaC2, is produces when heating up a mixture of lime and Carbon at 3000ºC in an electric oven and it is an acetylate which produces acetylene by hydrolysis. The acetylene is the base material of a great number of important chemicals for the organic industrial chemistry. The pure Calcium Carbonate occurs in two crystalline forms: calcite, hexagonal shaped, which possesses birrefringent properties, and aragonite, rhombohedric. The natural Carbonates are the most abundant Calcium minerals. The Iceland spar and the calcite are essentially pure Carbonate forms, whilst the marble is impure and much more compact, reason why it can be polished. It’s very demanded as construction material. Although the Calcium Carbonate is very little soluble in water, it is quite soluble if the water contains dissolved Carbon dioxide, for in these solutions it forms biCarbonate when dissolving. This fact explains the cave formation, where the lime stone deposits have been in contact with acid waters. The Calcium halogenures include phosphorescent fluoride, which is the Calcium compound more abundant and with important applications in spectroscopy. The Calcium Chloride possesses, in the anhydric form, great deliquescence capacity, which makes it useful as industrial dehydrating agent and as sand whirl control factor in roads. Calcium hypochlorite (whitening powder) is produced in the industry when passing Chlorine through a lime solution, and has been used as a whitening agent and as water purifier. The dehydrated Calcium sulphate is the mineral gypsum, constitutes the bigger portion of Portland concrete, and has been used to reduce the alkalinity of soils. Heating gypsum at high temperatures produces a Calcium sulphate hemihydrate, which is sold with the commercial name of Parisian stucco. Calcium in the environment Calcium is the fifth element and the third most abundant metal in the earth’s crust. The Calcium compounds account for 3.64% of the earth’s crust. The distribution of Calcium is very wide; it is found in almost every terrestrial area in the world. This element is essential for the life of plants and animals, for it is present in the animal’s skeleton, in tooth, in the egg’s shell, in the coral and in many soils. Seawater contains 0.15% of Calcium Chloride.
Calcium cannot be found alone in nature. Calcium is found
mostly as limestone, gypsum and fluorite. Stalagmites and
stalactites contain Calcium Carbonate.
Calcium is always present in every plant, as it is essential for its growth. It is contained in the soft tissue, in fluids within the tissue and in the structure of every animal’s skeleton. The vertebrate’s bones contain Calcium in the form of Calcium fluoride, Calcium Carbonate and Calcium phosphate.
|