|
The name Aluminum is derived from the ancient name for alum
(Potassium Aluminum sulphate), which was alumen (Latin, meaning bitter
salt). Aluminum was the original name given to the element by Humphry
Davy but others called it Aluminum and that became the accepted name
in Europe. However, in the USA the preferred name was Aluminum and when
the American Chemical Society debated on the issue, in 1925, it decided
to stick with Aluminum.
Aluminum is a soft and lightweight metal. It has a dull silvery
appearance, because of a thin layer of oxidation that forms quickly when it
is exposed to air. Aluminum is nontoxic (as the metal) nonmagnetic and
non-sparking.
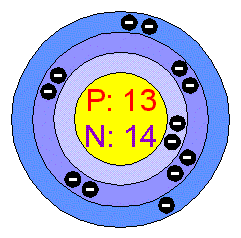
Aluminum has only one naturally occurring isotope, Aluminium-27,
which is not radioactive.
Applications
A silvery and ductile member of the poor metal group of elements,
Aluminum is found primarily as the ore bauxite and is remarkable for
its resistance to oxidation (Aluminum is actually almost always already
oxidized, but is usable in this form unlike most metals), its strength,
and its light weight. Aluminum is used in many industries to make
millions of different products and is very important to the world
economy. Structural components made from Aluminum are vital to the
aerospace industry and very important in other areas of transportation
and building in which light weight, durability, and strength are needed.
The use of Aluminum exceed that of any other metal except Iron. Pure
Aluminum easily forms alloys with many elements such as
Copper, Zinc,
Magnesium, Manganese and
Silicon.
Nearly all modern mirrors are made using a thin reflective coating
of Aluminum on the back surface of a sheet of float glass. Telescope
mirrors are also coated with a thin layer of Aluminum.
Other applications are electrical transmission lines, and packaging
(cans, foil, etc.).
Because of its high conductivity and relatively low price compared to
Copper, Aluminum was introduced for household electrical wiring to a
large degree in the US in the 1960s. Unfortunately problems on the
functioning were caused by its greater coefficient of thermal expansion
and its tendency to creep under steady sustained pressure, both
eventually causing loosening the connection; galvanic corrosion
increasing the electrical resistance.
The most recent development in Aluminum technology is the production of
Aluminum foam by adding to the molten metal a compound (a metal
hybrid), which releases Hydrogen gas. The molten Aluminum has to he
thickened before this is done and this is achieved by adding Aluminum
oxide or Silicon carbide fibers. The result is a solid foam which is
used in traffic tunnels and in space shuttle.
Aluminum in the environment
Aluminum is an abundant element in Earth's crust: it is believed
to be contained in a percentage from 7.5% to 8.1%. Aluminum is very rare in its free form.
Aluminum contribute greatly to the properties of soil, where it is
present mainly as insoluble Aluminum hydroxide.
Aluminum is a reactive metal and it is hard to extract it from its ore,
Aluminum oxide (Al2O3). Aluminum is among the
most difficult metals on earth to refine, the reason is that Aluminum
is oxidized very rapidly and that its oxide is an extremely stable
compound that, unlike rust on Iron, does not flake off. The very reason
for which Aluminum is used in many applications is why it is so hard to
produce.
Several gemstones are made of the clear crystal form of Aluminum
oxide known as corundum. The presence of traces of other metals creates
various colors: Cobalt creates blues sapphires, and Chromium makes red
rubies. Both these are now easy and cheap to manufacture artificially.
Topaz is Aluminum silicate coloured yellow by traces of Iron.
Recovery of this metal from scrap (via recycling) has become an
important component of the Aluminum industry. Industrial production
world-wide of new metal is around 20 million tons per year, and a
similar amount is recycled. Known reserves of ores are 6 billion tones.
Aluminum is one of the most widely used
metals and also one of the most frequently found compounds in the
earth's crust. Due to these facts, Aluminum is commonly known as
an innocent compound. But still, when one is exposed to high
concentrations, it can cause health problems. The water-soluble
form of Aluminum causes the harmful effects, these particles are
called ions. They are usually found in a solution of Aluminum in
combination with other ions, for instance as Aluminum Chlorine.
The uptake of Aluminum can take place through food, through
breathing and by skin contact. Long lasting uptakes of significant
concentrations of Aluminum can lead to serious Health effects,
such as:
- Damage to the central nervous system
- Dementia
- Loss of memory
- Listlessness
- Severe trembling
Aluminum is a risk in certain working envIronments, such as
mines, where it can be found in water. People that work in
factories where Aluminum is applied during production processes
may endure lung problems when they breathe in Aluminum dust.
Aluminum can cause problems for kidney patients when it enters
the body during kidney dialyses.
Inhalation of finely divided Aluminum
and Aluminum oxide powder has been reported
as a cause of pulmonary fibrosis and lung damage. This effect,
know as Shaver’s Disease, is complicated by
the presence in the inhaled air of silica and
oxides of Iron. May also be implicated in Alzheimer’s disease.
The effects of Aluminum have drawn our attention, mainly due to the
acidifying problems. Aluminum may accumulate in plants and cause health
problems for animals that consume these plants.
The concentrations of Aluminum appear to be highest in acidified lakes.
In these lakes the number of fish and amphibians is declining due to
reactions of Aluminum ions with proteins in the gills of fish and the
embryo's of frogs.
High Aluminum concentrations do not only cause effects upon fish, but
also upon birds and other animals that consume contaminated fish and
insects and upon animals that breathe in Aluminum through air. The
consequences for birds that consume contaminated fish are eggshell
thinning and chicks with low birth-weights. The consequences for animals
that breathe in Aluminum through air may be lung problems, weight loss
and a decline in activity.
Another negative Environmental effect of Aluminum is that its ions can
react with phosphates, which causes phosphates to be less available to
water organisms.
High concentrations of Aluminum may not only be found in acidified
lakes and air, but also in the groundwater of acidified soils. There are
strong indications that Aluminum can damage the roots of trees when it
is located in groundwater.
|