|
 |
|
 |
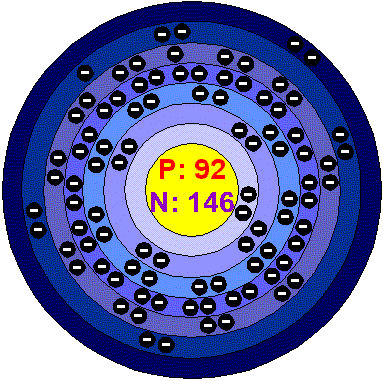
UraniumUranium is a hard, dense, malleable, ductile, Silver-white, radioactive metal. Uranium metal has very high density. When finely divided, it can react with cold water. In air it is coated by Uranium oxide, tarnishing rapidly. It is attacked by steam and acids. Uranium can form solids solutions and intermetallic compounds with many of the metals. Applications Uranium gained importance with the development of practical uses of nuclear energy. Depleted Uranium is used as shelding to protect tanks, and also in bullets and missiles. The first atomic bomb used in warfare was an Uranium bomb. This bomb contained enough of the uramium-235 isotope to start a runaway chain reaction which in a fraction of a second caused a large number of the Uranium atoms to undergo fission, there by releasing a fireball of energy. The main use of Uranium in the civilian sector is to fuel commercial nuclear power plants. This require Uranium to be enriched with the Uranium-235 isotope and the chain reaction to be controlled so that the energy is released in a more manageable way. The isotope Uranium 238 is used to estimate the age of the earliest igneous rocks and for other types of radiometric dating. Phosphate fertilizers are made from material typically high in Uranium, so they usually contain high amounts of it. Uranium in the environment Although Uranium is radioactive, it is not particularly rare. It is widely spread throughout the environment and so it is impossible to avoid Uranium. Uranium can be found naturally in the envIronment in very small amounts in rocks, soil, air and water. Humans add Uranium metals and compounds, because they are released during mining and milling processes. In air the Uranium concentrations are very low. Even at higher than usual concentrations in air, there is so little Uranium present per cubic meter that less than one atom transfers every day. In water most of the Uranium is dissolved Uranium that derives from rocks and soil that the water runs over. Some of the Uranium is suspended, so that the water gets a muddy texture. Only a very small part of Uranium in water settles from air. The amounts of Uranium in drinking water are generally very low. Uranium is found in soils in varying concentrations that are usually very low. Humans add Uranium to the soil through industrial activities. Erosion of tailing from mines and mills may cause larger amounts of Uranium to be released into the environment. Health effects of UraniumPeople always experience exposure to a
certain amount of Uranium from food, air, soil and water, as it is
naturally present in all these components. Food, such as root
vegetables, and water will provide us with small amounts of
natural Uranium and we will breathe in minimal concentrations of
Uranium with air. The concentrations of Uranium in seafood are
usually so low that they can be safely ignored. Effects of Uranium on the environmentUranium is a radioactive material that is very reactive. As a result it cannot be found in the environment in its elemental form. Uranium compounds that have consisted during reactions of Uranium with other elements and substances dissolve in water to their own extend. The water-solubility of a Uranium compound determines its mobility in the environment, as well as its toxicity. While Uranium itself is not particularly
dangerous, some of its decay products do pose a threat,
expecially Radon, which can build up in confined spaces such as
basements. |