|
 |
|
 |
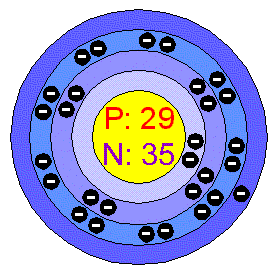
CopperCopper is a reddish metal with a face-centered cubic crystalline structure. It reflects red and orange light and absorbs other frequencies in the visible spectrum, due to its band structure, so it as a nice reddish colour. It is malleable, ductile, and an extremely good conductor of both heat and electricity. It is softer than Iron but harder than Zinc and can be polished to a bright finish. It is found in group Ib of the periodic table, together with Silver and Gold. Copper has low chemical reactivity. In moist air it slowly forms a greenish surface film called patina; this coating protects the metal from further attack. Applications Most Copper is used for electrical equipment (60%); construction,
such as roofing and plumbing (20%); industrial machineri, such as heat
exchangers (15%) and alloys (5%). The main long established Copper
alloys are bronze, brass (a Copper-Zinc alloy),
Copper-tin-Zinc, which was strong enough to make
guns and cannons, and was known as gun metal, Copper and
nichel, known as cuproNickel, which was the
preferred metal for low-denomination coins. Copper in the environment Copper is a very common substance that occurs naturally in the environment and spreads through the environment through natural phenomena. Humans widely use Copper. For instance it is applied in the industries and in agriculture. The production of Copper has lifted over the last decades and due to this Copper quantities in the environment have expanded. The world's Copper production is still
rising. This basically means that more and more Copper ends up in
the environment. Rivers are depositing sludge on their banks that
is contaminated with Copper, due to the disposal of
Copper-containing wastewater. Copper enters the air, mainly
through release during the combustion of fossil fuels. Copper in
air will remain there for an eminent period of time, before it
settles when it starts to rain. It will than end up mainly in
soils. As a result soils may also contain large quantities of
Copper after Copper from the air has settled. Most Copper compounds will settle and be bound to either water sediment or soil particles. Soluble Copper compounds form the largest threat to human health. Usually water-soluble Copper compounds occur in the environment after release through application in agriculture. World production of Copper amounts to 12 million tonnes a year and
exploitable reserves are aroun 300 million tonnes, which are expected to
last for only another 25 years. About 2 million tonnes a year are
reclaimed by recycling. Today Copper is mined as major deposists in
Chile, Indonesia, USA, Australia and Canada, which together account for
around 80% of the world's Copper. The main ore is a yellow Copper-Iron
sulfide called chalcopyrite (CuFeS2). Health effects of CopperRoutes of exposition Copper can be found in many kinds of food, in drinking water and in air. Because of that we absorb eminent quantities of Copper each day by eating, drinking and breathing. The absorption of Copper is necessary, because Copper is a trace element that is essential for human health. Although humans can handle proportionally large concentrations of Copper, too much Copper can still cause eminent health problems. Copper concentrations in air are usually quite low, so that exposure to Copper through breathing is negligible. But people that live near smelters that process Copper ore into metal do experience this kind of exposure. People that live in houses that still have Copper plumbing are exposed to higher levels of Copper than most people, because Copper is released into their drinking water through corrosion of pipes. Occupational exposure to Copper often occurs. In the work place envIronment Copper contagion can lead to a flu-like condition known as metal fever. This condition will pass after two days and is caused by over sensitivity. Effects Long-term exposure to Copper can cause irritation of the nose,
mouth and eyes and it causes headaches, stomachaches, dizziness,
vomiting and diarrhoea. Intentionally high uptakes of Copper may
cause liver and kidney damage and even death. Whether Copper is
carcinogenic has not been determined yet. Industrial exposure to Copper fumes, dusts, or mists may result in metal fume fever with atrophic changes in nasal mucous membranes. Chronic Copper poisoning results in Wilson’s Disease, characterized by a hepatic cirrhosis, brain damage, demyelination, renal disease, and Copper deposition in the cornea. Environmental effects of Copper
Copper does not break down in the environment and because of that it can accumulate in plants and animals when it is found in soils. On Copper-rich soils only a limited number of plants has a chance of survival. That is why there is not much plant diversity near Copper-disposing factories. Due to the effects upon plants Copper is a serious threat to the productions of farmlands. Copper can seriously influence the proceedings of certain farmlands, depending upon the acidity of the soil and the presence of organic matter. Despite of this, Copper-containing manures are still applied. Copper can interrupt the activity in soils, as it negatively influences the activity of microrganisms and earthworms. The decomposition of organic matter may seriously slow down because of this. When the soils of farmland are polluted with Copper, animals will absorb concentrations that are damaging to their health. Mainly sheep suffer a great deal from Copper poisoning, because the effects of Copper are manifesting at fairly low concentrations. |