|
 |
|
 |
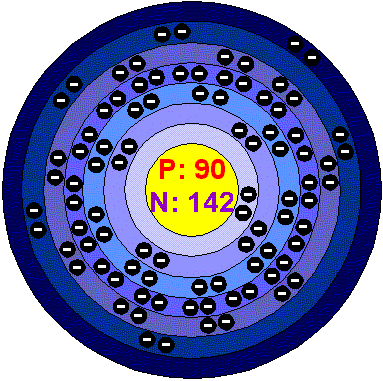
ThoriumWhen pure, Thorium is a silvery white metal which is air-stable and retains its lustre for several months. When contaminated with the oxide, Thorium slowly tarnishes in air, becoming grey and finally black. Thorium oxide has a melting point of 3300°C, the highest of all oxides. Only a few elements, such as Tungsten, and a few compounds, such as Tantalum carbide, have higher melting points. Thorium is slowly attacked by water, but does not dissolve readily in most common acids, except hydrochloric. Powdered Thorium metal is often pyrophoric and should be carefully handled. When heated in air, Thorium turnings ignite and burn brilliantly with a white light. Thorium was discovered by Jöns Jacob Berzelius, a Swedish chemist, in 1828. He discovered it in a sample of a mineral that was given to him by the Reverend Has Morten Thrane Esmark, who suspected that it contained an unknown substance. Esmark's mineral is now known as thorite (ThSiO 4 ). Thorium is named for Thor, the Scandinavian god of war. It is found in thorite and thorianite in New England (USA) and other sites. Thorium is a source of nuclear power. There is probably more untapped energy available for use from Thorium in the minerals of the earth's crust than from combined Uranium and fossil fuel sources. Much of the internal heat the earth has been attributed to Thorium and Uranium. Applications Until the inherent dangers associated with its radioactivity were realized, Thorium and its compounds found some important retail uses, the best known of which was in gas mantles, and in toothpaste. Thorium is still used as an alloying element in Magnesium, to coat Tungsten wire used in electronic equipment, to control the grain size of Plutonium used for electric lamps. in the manufacture of refractory materials for the metallurgical industries. Thorium oxide is used for high-temperatore laboratory crucibles, it is added to glass to create glasses with an high refractive index and low dispersion (lenses for cameras and scientific instruments). Like Uranium, Thorium could be a source if nuclear fuel. Thorium can be burnt in a nuclear reactor, without generating Plutonium. Uranium and Thorium has been used to date hominid fossils. Thorium in the environment Thorium is surprisingly abundant in the Earth's crust, being almost as abundant as lead and three times more abundant than Uranium. It is found in small amounts in most rocks and soils. Granitile contains up to 80 ppm of Thorium. Because Thorium oxide is highly insoluble, very little of this element circulates through the environment. Thorium occurs naturally as the minerals thorite, uranothorite, thorianite, it is a major component of monazite and it is present in significant amounts in the minerals zircon, titanite, gadolinite and betafite. World production of Thorium exceed 30.000 tonnes per year. Known reserves exceed 3 million tonnes. The amounts of Thorium in the environment may be incidentally increased due to accidental releases of Thorium processing plants. Health effects of ThoriumPeople will always be exposed to small
amounts of Thorium through air, food and water, because it is
found nearly everywhere on earth. Environmental effects of ThoriumEnvironmental stability: Thorium will slowly react with water, Oxygen, and other compounds to form a wide variety of Tungsten compounds. Effect of material on plants or animals: Due to the product size and the product’s form, no unusual Environmental effects are expected from these products; however, large releases of Thorium may be harmful to contaminated plants and animals. Effect of chemical on aquatic life: Due to the product size and the product’s form, these products are not anticipated to cause adverse effects on aquatic life; however, large releases of Thorium into a body of water may be harmful to aquatic plants and animals. Waste disposal must be in accordance with appropriate federal, state, and local regulations. These products, if unaltered by use, may be disposed of by treatment at a permitted facility or as advised by your local hazardous waste regulatory authority. All work practices must be aimed at eliminating Environmental contamination. |