|
 |
|
 |
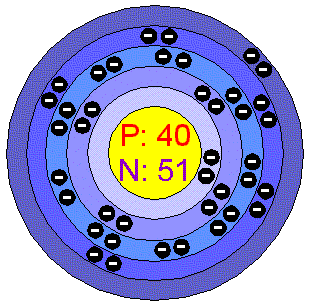
Z irconiumZirconium is a very strong, malleable, ductile, lustrous Silver-gray metal. Its chemical and physical properties are similar to those of Titanium. Zirconium is extremely resistant to heat and corrosion. Zirconium is lighter than steel and its hardness is similar to Copper. When it is finely divided, the metal can spontaneously ignite in air, especially at high temperatures. Zirconium powder is black and is regarded as very dangerous fire hazard. Zirconium does not dissolve in acids and alkalis. Applications Z irconium is u sed in alloys such as zircaloy, which is used in nuclear applications since it does not readily absorb neutrons. Also used in catalytic converters, percussion caps and furnace bricks. Baddeleyite and impure Zirconium (zirconia) are used in lab crucibles. The major end uses of zircon (ZrSiO4) are refractories, ceramic opacification and foundry sands. Zircon is also marketed as a natural gemstone used in jewelry. The metal also has many other uses, among them in photographic flashbulbs and surgical instruments, to make the glass for television, in the removal of residual gases from electronic vacuum tubes, and as a hardening agent in alloys, especially steel. The paper and packaging industries are finding that Zirconium compounds make good surface coatings because they have excellent water resistance and strength. Zirconium in the environment Zirconium is not a particularly rare element but because its most common mineral, zircon, is highly resistant to weatering it is only slightly mobile in the environment. Zirconium is more than twice as abundant as Copper and Zinc and more than 10 times more abundant than lead. The chief ores are zircon (ZrSiO4), which is mined in Australia, USA and Sri Lanka, and baddeleyite (Zirconium oxide ZrO2) which is mined in Brasil. World production is in excess of 900.000 tonnes per year of zircon, and 7000 tonnes of the metal are produced. The estimated reserves exceed a billion tonnes. Australia, South Africa, India, Sri Lanka and the USA have vast deposits of zircon and zirconia sands. Zirconium and its salts generally have low systemic toxicity. The estimated dietary intake is about 50 microg. Most passes through the gut without being adsorbed, and that which is adsorbed tends to accumulate slightly more in the skeleton than in tissue. Zirconium 95 is one of the radionuclides involved in atmospheric testing of nuclear weapons. It is among the long-lived radionuclides that have produced and will continue to produce increased cancers risk for decades and centuries to come.
|