|
 |
|
 |
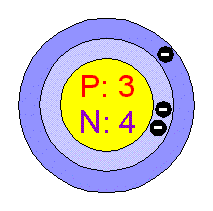
LithiumLithium is the first of the alkalis in the periodic table. In nature itís found like a mixture of the isotopes Li6 and Li7. Itís the lightest solid metal, itís soft, silvery-white, with a low melting point and reactive. Many of its physical and chemical properties are more similar to those of the alkaline earth metals than to those of its own group.
Between the most significant properties of lithium we find its
high specific heat (calorific capacity), the huge temperature interval
in the liquid state, high termic conductivity, low viscosity and very
low density. Metallic lithium is soluble in short chain aliphatic
amines, like etilamine. Itís insoluble in hydroCarbons. Applications
The main lithium compound is the lithium hydroxide. Itís a
white powder; the manufactured material is monohydrate lithium
hydroxide. The Carbonate can be used in the pottery industry and in
medicine as an antidepressant. The bromine and the lithium Chloride both
form concentrated brine, which have the property of absorbing the
humidity in a wide interval of temperature; these brines are used in the
manufactured air conditioning systems.
Lithium in the environment Like all alkali metals, lithium reacts easily in water and does not
occur freely in nature due to its activity, Lithium is a
moderately abundant element and its present in The Earthís crust in 65
ppm (parts per million). This situates lithium below Nickel,
Copper, and Tungsten and over Cerium and tin, referring to abundance. Health effects of LithiumEffects of exposure to Lithium: Fire: Flammable. Many reactions may cause fire or explosion. Gives off irritating or toxic fumes (or gases) in a fire. Explosion: Risk of fire and explosion on contact with combustible substances and water. Inhalation: Burning sensation. Cough. Laboured breathing. Shortness of breath. Sore throat. Symptoms may be delayed. Skin: Redness. Skin burns. Pain. Blisters. Eyes: Redness. Pain. Severe deep burns. Ingestion: Abdominal cramps. Abdominal pain. Burning sensation. Nausea. Shock or collapse. Vomiting. Weakness. Effects of short-term exposure: The substance is corrosive to the eyes, the skin and the respiratory tract. Corrosive on ingestion. Inhalation of the substance may cause lung oedema. The symptoms of lung oedema often do not become manifest until a few hours have passed and they are aggravated by physical effort. Rest and medical observation is therefore essential. Immediate administration of an appropriate spray, by a doctor or a person authorized by him/her, should be considered. Routes of exposure: The substance can be absorbed into the body by inhalation of its aerosol and by ingestion. Inhalation risk: Evaporation at 20įC is negligible; a harmful concentration of airborne particles can, however, be reached quickly when dispersed. Chemical dangers: Heating may cause violent combustion or explosion. The substance may spontaneously ignite on contact with air when finely dispersed. Upon heating, toxic fumes are formed. Reacts violently with strong oxidants, acids and many compounds (hydroCarbons, halogens, halons, concrete, sand and asbestos) causing fire and explosion hazard. Reacts violently with water, forming highly flammable Hydrogen gas and corrosive fumes of lithium hydroxide. Environmental effects of LithiumMetallic lithium will react with Nitrogen, Oxygen, and water vapor in air. Consequently, the lithium surface becomes coated with a mixture of lithium hydroxide (LiOH), lithium Carbonate (Li 2 CO 3 ), and lithium nitride (Li 3 N). Lithium hydroxide represents a potentially significant hazard because it is extremely corrosive. S pecial attention should be given to water organisms. |