|
 |
|
 |
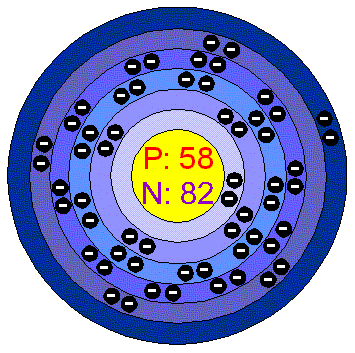
CeriumCerium is a malleable, soft, ductile, Iron-grey metal, slightly harder than lead. It is very reactive: it tarnishes readily in the air, it oxidizes slowly in cold water and rapidly in hot water. It dissolves in acids. It can burn when heated or scratched with a knife. Applications The metal is used as a core for the Carbon electrodes of arc lamps,
for incandescent mantles for gas lighting. Cerium is used in Aluminium
and Iron alloys, in stainless steel as a precipitation hardening agent,
to make permanent magnets. Cerium oxide is part of the catalyst of
catalytic converters used to clean up exhaust vehicles, it also
catalyzes the reduction of Nitrogen oxides (NOx) to Nitrogen
gas.
All new cars are now equipped with catalytic conveter which consist in a
ceramic or metal substrate, a coating of Aluminium and Cerium oxides and
a layer of finely dispersed metal such as Platinum
or Rhodium, which is the active surface. Cerium in the environment Cerium is the most abundant of the rare earth elements. It makes up about 0.0046 % of the Earth's crust by weight. Cerium comes mainly from the major lanthanide ores but some is obtained from perovskite, a Titanium mineral and allanite, both of which can have enough Cerium to make them viable sources. Production amounts to 23.000 tonnes a year, but this amount is likely to increase since more and more Cerium is used nowadays. Health effects of CeriumCerium is one of the rare chemicals, that can be found in houses in equipment such as colour televisions, fluorescent lamps, energy-saving lamps and glasses. All rare chemicals have comparable properties. Cerium is mostly dangerous in the working envIronment, due to the fact that damps and gasses can be inhaled with air. This can cause lung embolisms, especially during long-term exposure. Cerium can be a threat to the liver when it accumulates in the human body. Cerium has no know biological role, but it has been noted that Cerium salts stimulate metabolism. Environmental effects of CeriumCerium is dumped in the environment in many
different places, mainly by petrol-producing industries. It can
also enter the environment when household equipment is thrown
away. Cerium will gradually accumulate in soils and water soils
and this will eventually lead to increasing concentrations in
humans, animals and soil particles. Thanks to its use in catalytic converters Cerium is slowly improving the atmosphere of cities, or wherever diesel engines operates. Diesel engines emits particulates, Carbon particles only a few micrometers in diameter. One way to reduce particulates emissions is trap them in a ceramic filter and then burn them off. If a little Cerium oxide is added to the fuel itself, it will catalyse the burning of the particulates and eliminate them. |