|
|
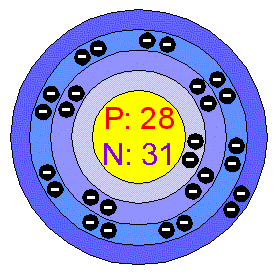
NickelNickel is silvery-white. hard, malleable, and ductile metal. It is of the Iron group and it takes on a high polish. It is a fairly good conductor of heat and electricity. In its familiar compounds Nickel is bivalent, although it assumes other valences. It also forms a number of complex compounds. Most Nickel compounds are blue or green. Nickel dissolves slowly in dilute acids but, like Iron, becomes passive when treated with nitric acid. Finely divided Nickel adsorbs Hydrogen. Applications The major use of Nickel is in the preparation of alloys. Nickel alloys are
characterized by strength, ductility, and resistance to
corrosion and heat. About 65 % of
the Nickel consumed in the Western World is used to make stainless
steel, whose composition can vary but is tipycally Iron with aroun 18% Chromium and 8% nichel.
12 % of all the nichel consumed goes into superalloys. The remaining 23%
of consumption is divided between alloy steels, rechargeable batteries,
catalysts and other chemicals, coinage, foundry products, and plating. Nickel in the environment Most nichel on Earth is inacessible because it is locked away in the
planet's Iron-Nickel molten core, which is 10 % Nickel. The total amount
of Nickel dissolved in the sea has been calculated to be around 8
billion tonnes. Organic matter has a strong ability to absorb the metal
which is why coal and oil contain considerable amounts. The nichel
content in soil can be as low as 0.2 ppm or as high as 450 ppm in some
clay and loamy soils. The average is around 20 ppm. Nickel occurs in
some beans where it is an essential component of some enzymes. Another
relatively rich source of Nickel is tea which has 7.6 mg/kg of dried
leaves. Health effects of NickelNickel is a compound that occurs in the envIronment only at very low levels. Humans use Nickel for many different applications. The most common application of Nickel is the use as an ingredient of steal and other metal products. It can be found in common metal products such as jewellery. Foodstuffs naturally contain small amounts of Nickel. Chocolate
and fats are known to contain severely high quantities. Nickel
uptake will boost when people eat large quantities of vegetables
from polluted soils. Plants are known to accumulate Nickel and as
a result the Nickel uptake from vegetables will be eminent.
Smokers have a higher Nickel uptake through their lungs. Finally,
Nickel can be found in detergents.
Nickel fumes are respiratory irritants
and may cause pneumonitis. Exposure to Nickel
and its compounds may result in the development of a dermatitis known as “Nickel itch” in
sensitized individuals. The first symptom is usually
itching, which occurs up to 7 days before skin eruption occurs. The primary skin eruption is
erythematous, or follicular, which may be
followed by skin ulceration. Nickel sensitivity, once acquired,
appears to persist indefinitely. Effects of Nickel on the environmentNickel is released into the air by power
plants and trash incinerators. It will than settle to the ground
or fall down after reactions with raindrops. It usually takes a
long time for Nickel to be removed from air. Nickel can also end
up in surface water when it is a part of wastewater streams. |