|
 |
|
 |
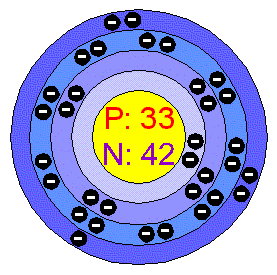
Arsenic
Arsenic appears in three allotropic forms: yellow, black and grey; the
stable form is a Silver-gray, brittle crystalline solid. It tarnishes
rapidly in air, and at high temperatures burns forming a white cloud of
Arsenic trioxide. Arsenic is a member of group Va of the
periodic table, which combines readily with many elements. Applications
Arsenic compounds are used in making special types of glass, as a wood
preservative and, lately, in the semiconductor Gallium arsenade, which has the ability to convert electric current
to laser light. Arsine gas AsH3, has become an important
dopant gas in the microchip industry, although it requires strict
guidelines regarding its use because it is extremely toxic. Arsenic in the environment Arsenic can be found naturally on earth in small concentrations. It occurs in soil and minerals and it may enter air, water and land through wind-blown dust and water run-off. Arsenic in the atmosphere comes from various sources: vulcanoes release about 3000 tonnes per year and microorganisms release volatile methylarsines to the extent of 20.000 tonnes per year, but human activity is responsible for much more: 80.000 tonnes of Arsenic per year are released by the burning of fossil fuels.
Despite its notoriety as a deadly poison, Arsenic is an essential trace
element for some animals, and maybe even for humans, although the
necessary intake may be as low as 0.01 mg/day. A little uncombined Arsenic occurs naturally as microcrystalline masses, found in Siberia, Germany, France, Italy, Romania and in the USA. Most Arsenic is found in conjuction with Sulfur in minerals such as arsenopyrite (AsFeS), realgar, orpiment and enargite. Non is mined as such because it is produced as a by-product of refining the ores of other metals, such as Copper and lead. World production of Arsenic, in the form of its oxide, is around 50.000 tonnes per year, far in excess of that required by industry. China is the chief exporting country, followed by Chile and Mexico. World resources of Arsenic in Copper and lead ores exceed 10 million tonnes. Health effects of ArsenicArsenic is one of the most toxic elements
that can be found. Despite their toxic effect, inorganic Arsenic
bonds occur on earth naturally in small amounts. Humans may be
exposed to Arsenic through food, water and air. Exposure may also
occur through skin contact with soil or water that contains
Arsenic. A lethal dose of Arsenic oxide is generally
regarded as 100 mg. Environmental effects of ArsenicThe Arsenic cycle has broadened as a consequence of human interference and due to this, large amounts of Arsenic end up in the environment and in living organisms. Arsenic is mainly emitted by the Copper producing industries, but also during lead and Zinc production and in agriculture. It cannot be destroyed once it has entered the environment, so that the amounts that we add can spread and cause Health effectsto humans and animals on many locations on earth. Plants absorb Arsenic fairly easily, so that high-ranking concentrations may be present in food. The concentrations of the dangerous inorganic Arsenics that are currently present in surface waters enhance the chances of alteration of genetic materials of fish. This is mainly caused by accumulation of Arsenic in the bodies of plant-eating freshwater organisms. Birds eat the fish that already contain eminent amounts of Arsenic and will die as a result of Arsenic poisoning as the fish is decomposed in their bodies. |