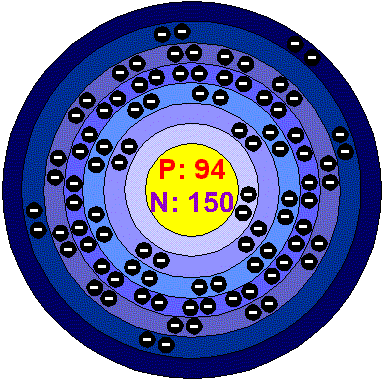
|
Plutonium was discovered in 1941 by Dr. Glenn
T. Seaborg and Edwin McMillan, Kennedy, and Wahl by deuteron
bombardment of Uranium in the 60-inch cyclotron of the
Berkeley Radiation Laboratory
at the University of California, Berkeley,
but the discovery was kept secret. It was named after the planet Pluto,
having been discovered directly after
Neptunium
.
(Pluto is the next planet out after Neptune).
The metal has a silvery appearance and takes on a yellow
tarnish when slightly oxidized. It is chemically reactive. A relatively
large piece of Plutonium is warm to the touch because of the energy
given off in alpha decay. Larger pieces will produce enough heat to boil
water. The metal readily dissolves in concentrated hydrochloric acid, hydroiodic acid, or
perchloric acid. The metal exhibits six allotropic
modifications having various crystalline structures. The densities of
these vary from 16.00 to 19.86 g/cm3.
The most important isotope
of Plutonium is 239Pu, with a half-life
of 24,200 years. Because of its short half-life, there are only
extremely tiny trace amounts of Plutonium naturally in Uranium
ores.
It is produced in extensive quantities in nuclear reactors from
natural Uranium: 238U(n, gamma) --> 239U--(beta) --> 239Np--(beta)
--> 239Pu. Fifteen isotopes of Plutonium are known.
Applications
Plutonium is a key fissile component in modern nuclear weapons ; care
must be taken to avoid accumulation of amounts of Plutonium which approach
critical mass , the amount of Plutonium which will self-generate a nuclear
reaction. Despite not being confined by external pressure as is required for a
nuclear weapon, it will nevertheless heat itself and break whatever confining
envIronment it is in. Shape is relevant; compact shapes such as spheres are to
be avoided.
Plutonium could also be used to manufacture radiological weapons. The Plutonium
isotope 238Pu is an alpha emitter with a half life of 87 years. These
characteristics make it well suited for electrical power generation for devices
which must function without direct maintenance for timescales approximating a
human life time. It is therefore used in RTGs such as those powering the Galileo
and Cassini space probes. Plutonium-238 was used on the Apollo-14 lunar flight
in 1971 to power seismic devices and other equipment left on the Moon, and it
was also the power supply of the two Voyager supercraft launched in 1977.
Plutonium-239 can also be used as a fuel in a new
generation of fast-breeder nuclear weapons, which burn a mixed oxide
(MOX) fuel consisting of Uranium and Plutonium.
Plutonium in the environment
Trace amounts of Plutonium are found
naturally in Uranium-rich ores. Humans produce most of the
existing Plutonium, in special nuclear reactors. Besides being
naturally present in very small amounts, Plutonium may also enter
the environment from releases of nuclear reactors, weapons
production plants, and research facilities. A major source of
Plutonium release is nuclear weapons testing.
Annual world production of Plutonium is probably in excess of 50
tonnes and there may be more than 1.000 tonnes of metal in storage,
either as bombs or as metal rods.
Plutonium is sometimes
described in media reports as the most toxic substance known to man, although
there is general agreement among experts in the field that this is incorrect. As
of 2003, there has yet to be a single human death officially attributed to
Plutonium exposure. Naturally-occurring Radium is about 200 times more
radiotoxic than Plutonium, and some organic toxins like Botulism toxin are
billions of times more toxic than Plutonium.
The alpha radiation it emits does not penetrate the
skin, but can irradiate internal organs when Plutonium is inhaled or ingested.
Extremely small particles of Plutonium on the order of micrograms can cause lung
cancer if inhaled into the lungs. Considerably larger amounts may cause acute
radiation poisoning and death if ingested or inhaled; however, so far, no human
is known to have died because of inhaling or ingesting Plutonium and many people
have measurable amounts of Plutonium in their bodies. Plutonium is a dangerous
substance that has been used in explosives for a long time. It is released into
the atmosphere primarily by atmospheric testing of nuclear weapons and by
accidents at weapon production sites. When Plutonium is released into the
atmosphere it will fall back onto earth eventually and end up in soils.
Exposure of humans to Plutonium is not likely, but sometimes it takes place as a
result of accidental releases during use, transport or disposal.
Because Plutonium has no gamma radiation, Health effectsare not likely to occur
while working with Plutonium, unless it is breathed in or swallowed somehow.
When people breathe it in, Plutonium may remain in the lungs or move to the
bones or organs. Generally it stays in the body for a long time and continually
exposes body tissues to radiation. After a few years this could result in the
development of cancer.
Furthermore, Plutonium may affect the ability to resist disease and the
radioactivity from Plutonium may cause reproductive failure.
Plutonium may enter surface water from accidental releases and
disposal of radioactive wastes. Soil can become contaminated with
Plutonium through fallout during nuclear weapons testing.
Plutonium moves slowly downwards in the soil, into the
groundwater.
Plants absorb low levels of Plutonium, but these levels are not
high enough to cause bio magnification of Plutonium up the food
chain, or accumulation in the bodies of animals.
|