|
 |
|
 |
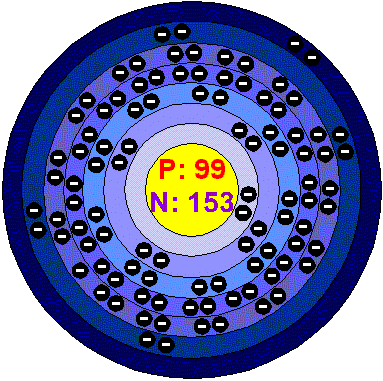
EinsteiniumEinsteinium is a member of the actinide series, it is metallic and radioactive, with no known uses. It is attacked by Oxygen, steam and acids but not by alkalis. It is named after Albert Einstein. It was the seventh transuranic element to be discovered. It was first identified in December 1952 by Albert Ghiorso at the University of California, Berkeley and another team headed by G.R. Choppin at Los Alamos. Both were examining debris from a nuclear weapon test of November, 1952. They discovered the isotope 253, which has a half-life of 20.5 days. In 1961, enough Einsteinium was produced to separate a macroscopic amount of isotope 253. This sample weighted about 0.01 mg and was measured using a special balance. The material produced was used to produce Mendelevium. Further Einsteinium has been produced at the Oak Ridge National Laboratory. Around 3 mg was created over a four year program of irradiation and then chemical separation from a starting 1 kg of Plutonium isotope. Fourteen isotopes of Einsteinium are now recognized. They have half-lives ranging from 2 seconds (257) up to 471 days (252). Applications There are, as yet, no known applications of einstenium. Einstenium in the environment Einstenium does not exist naturally on Earth today, but it has occurred in the past in nuclear reactor deposits. Health effects of einsteniumEinsteinium doesn’t occur naturally, and has not been found in the earth’s crust, so there is no reason to consider its health hazards. However it is higly dangerous because of the radiation it emits. Environmental effects of EinsteiniumEinsteinium doesn’t occur naturally, and has not been found in the earth’s crust, so there is no reason to consider its Environmental hazards. |