|
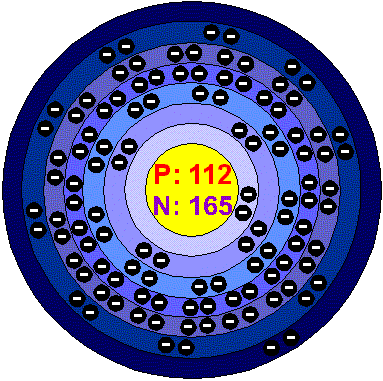 |
UnunbiumFrom its position in the periodic table, in group 12 below Mercury, this element should have the physical properties of a heavy metal and, were it long-enough lived, it should be possible for it to have two kind of chemistry, corresponding to oxidation states M(I) and M(II), with the latter more unstable. Following periodic trends, it is expected to be a liquid metal more volatile that Mercury.
Applications
Ununbium does not have any known application and little is known about it. Ununbium in the environment Ununbium is not found free in the environment, since it is a synthetic element. Health effects of UnunbiumAs it is so unstable, any amount formed would decompose to other elements so quickly that there’s no reason to study its effects on human health. Environmental effects of UnunbiumDue to its extremely short half-life (about 0.24 milliseconds ) , there’s no reason for considering the effects of Ununbium in the environment. |