|
 |
|
 |
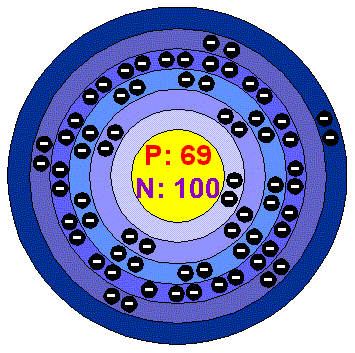
ThuliumThulium is a lanthanide element, it has a bright silvery-gray luster and can be cut by a knife. It is the least abundant of the rare earths and its metal is easy to work. It slowly tarnishes in air, but is more resistant to oxidation than most rare-earth elements. It also has some corrosion resistance in dry air and good ductility . Naturally occurring Thulium is made entirely of the stable isotope Tm-169. ApplicationsThe pure metal and compound have few commercial uses: because it is very rare and expensive and has little to offer, Thulium find little application outside chemical research. Thulium has been used to create lasers. When stable Thulium (Tm-169) is bombarded in a nuclear reactor it can later serve as a radiation source in portable X-ray devices. It also has potential use in ceramic magnetic materials called ferrites , which are used in microwave equipment. Thulium-doped Calcium sulphate has been used in personal radiation dosimeters because it can register, by its fluorescence, especially low levels.Thulium in the environment The element is never found in nature in pure form but it is found in small quantities in minerals with other rare earths. It is principally extracted from monazite , which contains about 0.007% of Thulium and bastnasite (about 0.0008%). The chief ores are in China, US, Brazil, India, Sri lanka and Australia. Reserves of Thulium are estimated to be about 100.000 tonnes. World production is about 50 tonnes per year as Thulium oxide. Thulim is the second rarest lenthanide element, after Promethium. Health effects of ThuliumSoluble Thulium salts are regarded as slightly toxic in taken in large amounts, but the soluble salts are completely not toxic. Effects of Thulium on the environmentThulium poses no enviromental threat to plants and animals. |