|
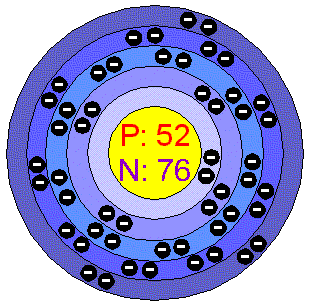 |
TelluriumTellurium is a semimetallic, lustrous, crystalline, brittle, Silver-white element. It is usually available as a dark grey powder, it has the properties both of the metals and the non metals. Tellurium forms many compounds corresponding to those of Sulfur and Selenium. When burned in air Tellurium has a greenish-blue flame and forms Tellurium dioxide as a result. Tellurium is uneffected by water or hydrochloric acid, but dissolves in nitric acid. Applications Tellurium is often used as an additive to steel and it is often alloyed to Aluminum, Copper, Lead or Tin. Tellurium is added to lead to improve its durability, strenght and resistence to corrosion. It can be used for cast Iron, ceramics, blasting caps, solar panels, chalcogenide glasses. When added to rubber, Tellurium speeds up the curing process and makes the product less susceptible to ageing and less likely to be affected by oil, which softens normal rubber. Tellurium in the environment Tellurium is present in coal at up to 2 ppm. This is probably the major source of this metal, which can be taken by plants from soil. Tellurium in plants can reach level as high as 6 ppm, although few food plants have more than 0.5 ppm and most have less than 0.05 ppm. Samples of uncombined Tellurium can be sometimes found, but they are extremely rare. There are some Tellurium minerals (calaverite, sylvanite, tellurite), but none is mined as a source of the element. World production is around 220 tonnes/year. Major producers are USA, Canada, Peru and Japan. The reserves of this element have not been assessed. Health effects of TelluriumFortunately, Tellurium compounds are encountered rarely by most people. They are teratogenic and should only be handled by competent chemists since ingestion in even small amounts causes dreadful smelling breath and appalling body odour.
Routes of exposure:
The substance can be absorbed
into the body by inhalation of its aerosol. Effects of short-term exposure: The aerosol of this substance irritates the eyes and the respiratory tract. The substance may cause effects on the liver and central nervous system. Exposure may result in garlic-like breath. Medical observation is indicated. Ingestion: Abdominal pain. Constipation. Vomiting. Chemical dangers: Upon heating, toxic fumes are formed. Reacts vigorously with halogens or interhalogens causing fire hazard. Reacts with Zinc with incandescence. Lithium silicide attacks Tellurium with incandescence. Combustible. Finely dispersed particles form explosive mixtures in air. Environmental effects of TelluriumNot harmful or readily rendered harmless by natural processes. When heated to decomposition, Tellurium Chloride may emit toxic fumes of Tellurium and Chlorine. |