|
 |
|
 |
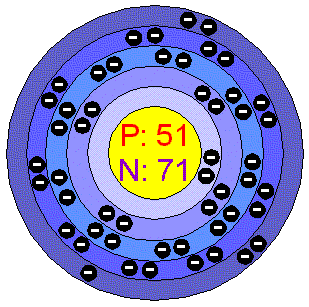
AntimonyAntimony si a semimetallic chemical element which can exist in two forms: the metallic form is bright, silvery, hard and brittle; the non metallic form is a grey powder. Antimony is a poor conductor of heat and electricity, it is stable in dry air and is not attacked by dilute acids or alkalis. Antimony and some of its alloys expand on cooling. Antimony has been known since ancient times. It is sometimes found free in nature, but is usually obtained from the ores stibnite (Sb 2 S 3 ) and valentinite (Sb 2 O 3 ). Nicolas Lémery, a French chemist, was the first person to scientifically study Antimony and its compounds. He published his findings in 1707. Antimony makes up about 0.00002% of the earth's crust. Applications Very pure Antimony is used to make certain types of semiconductor devices, such as diodes and infrared detectors. Antimony is alloyed with lead to increase lead's durability. Antimony alloys are also used in batteries, low friction metals, type metal and cable sheathing, among other products. Antimony compounds are used to make flame-proofing materials, paints, ceramic enamels, glass and pottery. The ancient Egyptians used Antimony, in the form of stibnite, for black eye make-up. Antimony in the environment Antimony occurs naturally in the environment. But it also enters the environment through several applications by humans. Antimony is an important metal in the world economy. Annual production is about 50.000 tonnes per year, with virgin materials coming mainly from china, Russia, Bolivia and South Africa. World reserves exceed 5 million tonnes. In Finland there is a deposit of elemental Antimony. Health effects of Antimony Especially people that work with Antimony can suffer the
effects of exposure by breathing in Antimony dusts. Human exposure
to Antimony can take place by breathing air, drinking water and
eating foods that contain it, but also by skin contact with soil,
water and other substances that contain it. Breathing in Antimony
that is bond to Hydrogen in the gaseous phase, is what mainly
causes the Health effects. Effects of Antimony on the environmentAntimony can be found in soils, waters and
air in very small amounts. Antimony will mainly pollute soils.
Through groundwater it can travel great distances towards other
locations and surface waters. |