|
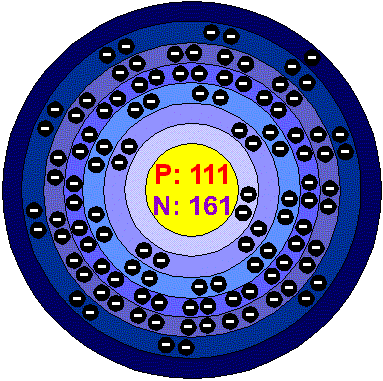
From its position in the periodic table, in group 11 below Gold, this element
should have the physical properties of a noble metal and, were it long enough
lived it should be possible to make compound of it although, like Gold, it might
be reluctant to form them. Its appearance is unknown, probably it is yellow or
orange metallic (like Gold).
Applications
Roentgenium does not have any known application
and little is known about it.
Roentgenium in the environment
Roentgenium is not found free in the environment, since it is a
synthetic element.
As
it is so unstable, any amount formed would decompose to other elements so
quickly that there’s no reason to study its effects on human health.
Due
to its extremely short half-life (about 1.5 milliseconds
)
,
there’s no reason for considering the effects of roetgenium in the
envIronment. |