|
|
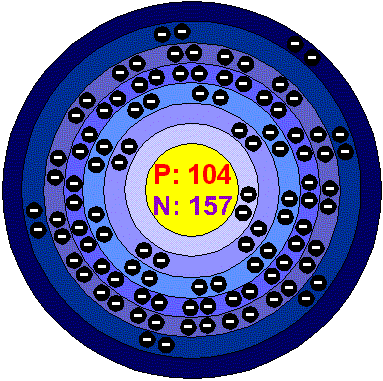
RutherfordiumRutherfordium is a highly radioactive chemical element. His most stable isotope Rf 265 has an half like of approximately 13 hours. Nine of its isotopes are known. Its predominant oxidation in +4 and complexes such as RfCl6 2- have been confirmed. Applications Rutherfordium does not have any application and little is known about it. Rutherfordium in the environment Rutherforium is not found free in the environment, since it is a synthetic element. Health effects of RutherfordiumAs it is so unstable, any amount formed would decompose to other elements so quickly that there’s no reason to study its effects on human health. Environmental effects of RutherfordiumDue to its extremely short half-life ( about 10 minutes) , there’s no reason for considering the effects of Rutherfordium in the environment. |