|
|
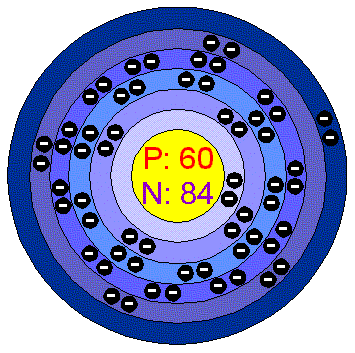
NeodymiumNeodimium is a lustrous silvery-yellow metal. It is very reactive and qickly turnishes in air and the coated formed does not protect the metal from further oxidation, so it must be stored away from contact with air. It reacts slowly with cold water and rapidly with hot. Applications Neodymium is one of the rare chemicals, that can be found in houses in equipment such as colour televisions, fluorescent lamps, energy-saving lamps and glasses. All rare chemicals have comparable properties. Nedymium is one of the several metals in alloys commonly used in lighter flints. The most important alloys is neodybium, Iron and Boron (NIB), found to make excellent permanent magnets. These magnets are part of modern vehicles components, used in computer data storing and in loudspeakers. Neodymium is used in coloring glasses (didymium glass) able to adsorb the yellow Sodium glare of the flame. This kind of glass is used to protect the eyes of welders. It is also used to tint glass attractive shades of purple. Neodymium in the environment Neodymium is the second most abundant of the rare-earth elements (after Cerium) an is almost as abundant as Copper. It is found in minerals that include all lanthanide minerals, such as monazite and bastnasite. The main areas are Brazil, China, USA, India, Sri Lanka and Australia. Reserves of neodybium are estimated to be 8 million tonnes, world production of neodybium oxide is about 7.000 tonnes a year. Health effects of NeodymiumThe amount of Neodymium in humans is quite
small and, although the metal has no biological role, it can be
effects on parts of the body: neodybium dust and salts are very
irritating to the eyes. Ingested neodybium salts are regarded as
only slightly toxic if they are soluble and non toxic if they
are insoluble. Environmental effects of NeodymiumNeodymium is dumped in the environment in
many different places, mainly by petrol-producing industries. It
can also enter the environment when household equipment is thrown
away. Neodymium will gradually accumulate in soils and water soils
and this will eventually lead to increasing concentrations in
humans, animals and soil particles. |