Chemical properties of Mendelevium - Health effects of Mendelevium - Environmental effects of Mendelevium
|
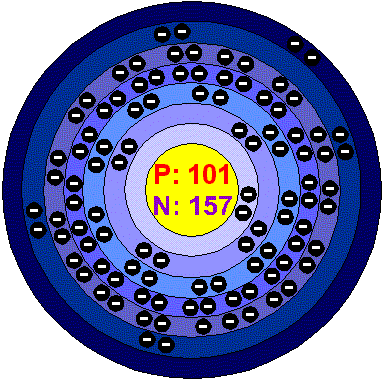 |
MendeleviumMendelevium is the first transferium element with the most stable isotope 258Md having a half-life of 52 days. It's chemical data are limited to its atomic number, its haf life and isotopes. Atomic weight of known isotopes range from 245 to 261. It is named after Dimitri Mendeleyev, who produced one of the first periodic tables. Applications The transferium elements have neither application nor economic role. Mendelevium in the environment The transferium elements do not exist in nature and they have very unstable nuclei, so they are quite hard to make and detect. Health effects of MendeleviumMendelevium doesn’t occur naturally, and has not been found in the earth’s crust, so there is no reason to consider its health hazards. Environmental effects of MendeleviumMendelevium doesn’t occur naturally, and has not been found in the earth’s crust, so there is no reason to consider its Environmental hazards. |