|
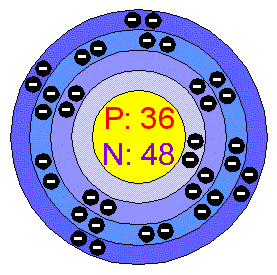
Krypton
is present in the air at about 1 ppm. The atmosphere of Mars contains a
little (about 0.3 ppm) of Krypton. It is characterised by its brilliant
green and orange spectral lines. The spectral lines of Krypton are
easily produced and some are very sharp. In 1960 it was internationally
agreed that the fundamental unit of length, the metre, should be defined
as 1 m = 1,650,763.73 wavelengths (in vacuo) of the orange-red line of
Kr-33.
Under normal conditions Krypton is colourless, odourless,
fairly expensive gas. Solid Krypton is a white crystalline substance
with a face-centered cubic structure which is common to all the
"rare gases". Krypton difluoride, KrF2, has been
prepared in gram quantities and can be made by several methods.
Other compounds are unstable, unless isolated in a matrix at very low
temperatures.
Applications
Krypton is used to fill electric lamp bulbs which are
filled with a mixture of Krypton and Argon, and
for various electronic devices. Krypton is also used in
photographic projection lamps, in very high-powered electric arc lights
used at airports and in some strobo-lamps, because it has an extremely
fast respons to an electric current.
A mixture of stable and unstable isotopes of Krypton
is produced by slow neutron fission of Uranium in nuclear reactors as
Kripron-85, its most stable isotope. It is used to detect leaks in
sealed containers, to excite phosphors in light sources with no external
source of energy, and in medicine to detect abnormal heart openings.
Kripton in the environment
Kripton might be one of the rarest gases in the
atmosphere, but in total there are more than 15 billion tonnes of this
metal circulating in the planet, of which only about 8 tonnes a year are
extracted, via liquid air.
Inhalation:
This gas is inert and is classified as a simple asphyxiant. Inhalation
in excessive concentrations can result in dizziness, nausea, vomiting,
loss of consciousness, and death. Death may result from errors in
judgment, confusion, or loss of consciousness which prevent self-rescue.
At low Oxygen concentrations, unconsciousness and death may occur in
seconds without warning.
The effect of simple asphyxiant gases is
proportional to the extent to which they
diminish the amount (partial pressure) of Oxygen in the air that is
breathed. The Oxygen may be diminished to 75% of it's normal percentage
in air before appreciable symptoms develop. This in turn requires the
presence of a simple asphyxiant in a concentration of 33% in the mixture
of air and gas. When the simple asphyxiant reaches a concentration of
50%, marked symptoms can be produced. A concentration of 75% is fatal in
a matter of minutes.
Symptoms:
The
first symptoms produced by a simple asphyxiant are rapid respirations
and air hunger. Mental alertness is diminished and muscular coordination
is impaired. Later judgment becomes faulty and all sensations are
depressed. Emotional instability often results and fatigue occurs
rapidly. As the asphyxia progresses, there may be nausea and vomiting,
prostration and loss of consciousness, and finally convulsions, deep
coma and death.
Krypton
is a rare atmospheric gas and as such is
non-toxic and chemically inert. The extreme cold temperature (-244oC)
will freeze organisms on contact, but no long term ecological effects
are anticipated.
Disposal considerations:
When disposal becomes necessary, vent gas slowly
to a well-ventilated out door location remote from personnel work areas
and building air intakes. Do not dispose of any residual gas in
compressed gas cylinders. Return cylinders to the supplier with residual
pressure, the cylinder valve tightly closed. Please be advised that
state and local requirements for waste disposal may be more restrictive
or otherwise different from federal regulations. Consult state and local
regulations regarding the proper disposal of this material.
|