|
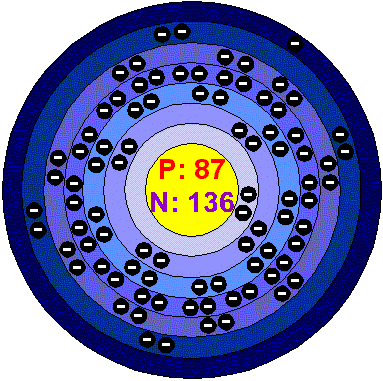 |
FranciumFrancium is extremely rare. Because of this its chemical and physical properties are not known. It has been studied by radiochemical techniques, which show that its most stable state is the ion Fr+. Francium is the least electronegative of all the known elements. Applications No use has been found for what little Francium can be produced. Francium in the environment Francium occurs naturally to a very limited extent in Uranium minerals. Nevertheless it has been estimated that there might be from 340 to 550 grams of Francium in the earth's crust at any one time. Francium is the second rarest element in the crust, after Astatine. Health effects of FranciumAs it is so unstable, any amount formed would decompose to other elements so quickly that there’s no reason to study its effects on human health. Environmental effects of FranciumDue to its extremely short half-life, there’s no reason for considering the effects of Francium in the environment. |