|
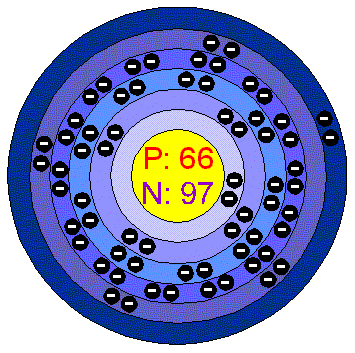 |
DysprosiumDysprosium is a lustrous, very soft, silvery metal. It is stable in air at room temperature even if it is slowly oxydized by Oxygen. It reacts with cold water and rapidly dissolves in acids. It forms several brightly coloured salts. Dysprosium's characteristics can be strongly affected by the presence of impurities. Applications Dysprosium is used in nuclear reactors as a cermet, a composite material made of ceramic and sintered metal, to make laser materials, nuclear reactor control rods, as sources of infrared radiation for studying chemical reactions. Another use in the field of radioactivity is in dosimeters for monitoring exposure to ionizing radiation. Dysprosium in the environment Dysprosium is one of the more abundant lanthanide elements and is more than twice as abundant as tin. Dysprosium is never encountered as the free element, but is found in many minerals. The most important ores are monazite and bastnasite. World production in around 100 tonnes per year. Health effects of DysprosiumDysprosium has no biological role. Soluble Dysprosium salts are mildly toxic by ingestion, while insoluble salts are non toxic. From toxicity tests on mice it was calculated that a dose of 500 grams or more would be needed to put a person's life at risk. Environmental effects of DysprosiumDysprosium poses no Environmental threat to plants and animals. |