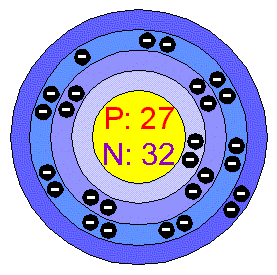
Cobalt
Cobalt is a hard ferromagnetic, Silver-white, hard, lustrous, brittle element. It is a member
of group VIII of the
periodic table. Like Iron, it can be magnetized. It is similar to
Iron and Nickel in its physical properties. The element is active
chemically, forming many compounds. Cobalt is stable in air and
unaffected by water, but is slowly attacked by dilute acids.
Applications
Cobalt is used in many alloys (superalloys for parts in gas turbine
aircrafr engines, corrosion resistant alloys, high-speed steels,
cemented carbides), in magents and magnetic recording media, as
catalysts for the petroleum and chemical industries, as drying agents
for paints and inks. Cobalt blue is an important part of artists'
palette and is used bu craft workers in porcelain, pottery, stained
glass, tiles and enamel jewellery. The radioactive isotopes, Cobalt-60,
is used in medical treatment and also to irradiate food, in order to
preserve the food and protect the consumer.
Cobalt in the enviroment
Most of the Earth's Cobalt is in its core. Cobalt is of relatively
low abundance in the Earth's crust and in natural waters, from which it
is precipitated as the highly insoluble Cobalt sulfine CoS.
Although the average level of Cobalt in soils is 8 ppm, there are soils
with as little as 0.1 ppm and others with as much as 70 ppm. In the
marine envIronment Cobalt is needed by
blue-green algae
(cyanobacteria) and other Nitrogen fixing organisms. Cobalt is not found
as a free metal and is generally found in the form of ores. Cobalt is
usually not mined alone, and tends to be produced as a by-product of
Nickel and Copper mining activities. The main ores of Cobalt are
Cobaltite, erythrite, glaucodot, and skutterudite. The world's major
producers of Cobalt are the Democratic Republic of the Congo, mainland
China, Zambia, Russia and Australia. It is also found in Finland,
Azerbaijan, and Kazakhstan.
World production is 17.000 tonnes per year.
As Cobalt is widely dispersed in the
envIronment humans may be exposed to it by breathing air, drinking
water and eating food that contains Cobalt. Skin contact with soil
or water that contains Cobalt may also enhance exposure.
Cobalt is not often freely available in the environment, but when
Cobalt particles are not bound to soil or sediment particles the
uptake by plants and animals is higher and accumulation in plants
and animals may occur.
Cobalt is beneficial for humans because it is a part of
vitamin
B12
, which is essential for human health. Cobalt is used to treat
anaemia with pregnant women, because it stimulates the production
of red blood cells. The total daily intake of Cobalt is variable
and may be as much as 1 mg, but almost all will pass through the
body unadsorbed, except that in vitamine B12.
However, too high concentrations of Cobalt may damage human
health. When we breathe in too high concentrations of Cobalt
through air we experience lung effects, such as asthma and
pneumonia. This mainly occurs with people that work with Cobalt.
When plants grow on contaminated soils they will accumulate very
small particles of Cobalt, especially in the parts of the plant we
eat, such as fruits and seeds. Soils near mining and melting
facilities may contain very high amounts of Cobalt, so that the
uptake by humans through eating plants can cause Health effects.
Health effectsthat are a result of the uptake of high
concentrations of Cobalt are:
- Vomiting and nausea
- Vision problems
- Heart problems
- Thyroid damage
Health effectsmay also be caused by radiation of radioactive
Cobalt isotopes. This can cause sterility, hair loss, vomiting,
bleeding, diarrhoea, coma and even death. This radiation is
sometimes used with cancer-patients to destroy tumors. These
patients also suffer from hair loss, diarrhea and vomiting.
Cobalt dust may cause an asthma-like
disease with symptoms ranging from
cough, shortness of breath and dyspnea to decreased pulmonary
function, nodular fibrosis, permanent
disability, and death. Exposure to Cobalt
may cause weight loss, dermatitis, and respiratory
hypersensitivity. LD 50
(oral, rat)- 6171 mg/kg. (LD50 = Lethal
dose 50 = Single dose of a substance that causes the death of 50%
of an animal population from exposure to the substance by any
route other than inhalation. LD50 is usually expressed as
milligrams or grams of material per kilogram of animal weight
(mg/kg or g/kg).)
Carcinogenicity- International Agency
for Research on Cancer (IARC) haslisted Cobalt and Cobalt
compounds within group 2B (agents which are possibly carcinogenic
to humans). ACGIH has placed Cobalt and inorganic compounds in category A3 (Experimental animal
carcinogen- the agent is carcinogenic in
experimental animals at a relatively high dose, by route(s),
histologic type(s), or by mechanism(s) that are
not considered relevant to worker exposure.)
Cobalt has been classified to be carcinogenic to experimental animals by the Federal Republic of
Germany.
Cobalt is an element that occurs naturally in
the environment in air, water, soil, rocks, plants and animals. It
may also enter air and water and settle on land through wind-blown
dust and enter surface water through run-off when rainwater runs
through soil and rock containing Cobalt.
Humans add Cobalt by releasing small amounts into the atmosphere
from coal combustion and mining, processing of Cobalt-containing
ores and the production and use of Cobalt chemicals.
The radioactive isotopes of Cobalt are not present in the
envIronment naturally, but they are released through nuclear power
plant operations and nuclear accidents. Because they have
relatively short half-lives they are not particularly dangerous.
Cobalt cannot be destroyed once it has entered the environment. It
may react with other particles or adsorb on soil particles or
water sediments. Cobalt will only mobilize under acidic
conditions, but ultimately most Cobalt will end up in soils and
sediments.
Soils that contain very low amounts of Cobalt may grow plants that
have a deficiency of Cobalt. When animals graze on these grounds
they suffer from lack of Cobalt, which is essential for them.
On the other hand, soils near mining and melting facilities may
contain very high amounts of Cobalt, so that the uptake by animals
through eating plants can cause Health effects. Cobalt will
accumulate in plants and in the bodies of animals that eat these
plants, but Cobalt is not known to bio magnify up the food chain.
Because of this fruits, vegetables, fish and other animals we eat
will usually not contain very high amounts of Cobalt. |