|
 |
|
 |
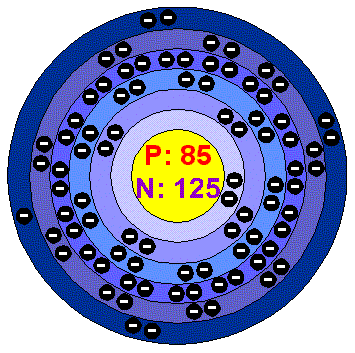
AstatineAstatine is a highly radioactive element and it is the heaviest known halogen. Its chemical properties are believed to be similar to those of Iodine. Is has been little researched because all its isotopes have short half lives. All that is known about the element has been estimated from knowing its position in the periodic table below Iodine and by studying its chemistry in extreme diluted solutions. Applications Astatine is never encountered outside nuclear facilities or research laboratories. Astatine in the environment Total world production of Astatine to date is estimated to be less than a millionth of a gram, and virtually all of this has now decayed away. Health effects of AstatineThe total amount of Astatine in the earth's crust at any particular time is less than 30 grams and only a few micrograms have ever been artificially produced. This, together with its short lifetime, leaves no reason for considering the effects of Astatine on human health. Astatine is studied in a few nuclear research laboratories where its high radioactivity requires special handling techniques and precautions.
Astatine is a halogen and
possibly accumulates in the thyroid like Iodine. From a chemical point
of view, one can speculate that its toxicity would mimic that of Iodine.
Environmental effects of AstatineAstatine does not occur to any significant extent in the biosphere and so normally never presents a risk. |