|
 |
|
 |
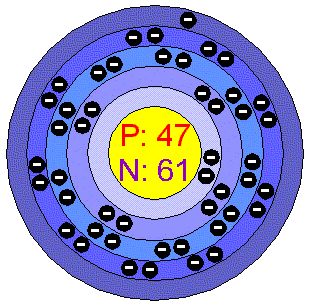
SilverPure Silver is nearly white, lustrous, soft, very ductile, malleable, it is an excellent conductor of heat and electricity. It is not a chemically active metal, but it is attacked by nitric acid (forming the nitrate) and by hot concentrated Sulfuric acid. It has the highest electrical conductivity of all metals, but its greater cost has prevented it from being widely used for electrical purposes. Silver is almost always monovalent in its compounds, but an oxide, a fluoride, and a sulfide of divalent Silver are known. It does not oxidize in air but reacts with the Hydrogen sulfide present in the air, forming Silver sulfide (tarnish). This is why Silver objects need regular cleaning. Silver is stable in water. Applications The principal use of Silver is as a precious metal and its halide
salts, especially Silver nitrate, are also widely used in photography.
The major outlets are photography, the electrical and electronic
industries and for domestic uses as cutlery, jewellery and mirrors. Silver in the environment Silver levels in soil are not usually high except in mineral-rich areas when they can sometimes be as much as 44 ppm. Plants can absorb Silver and measured levels come in the range 0.03-0.5 ppm. Metallic Silver occurs naturally as crystals, but more generally as a
compact mass; there are small deposits in Norway, Germany and Mexico.
The chief Silver ores are acanthite mined in Mexico, Bolivia and
Honduras, and stephanite, mined in Canada. However Silver is mostly
obtained as a byproduct in the refining of other metals. Soluble Silver salts, specially AgNO3, are lethal in concentrations of up to 2g (0.070 oz). Silver compounds can be slowly absorbed by body tissues, with the consequent bluish or blackish skin pigmentation (argiria). Eye contact: may cause severe corneal injury if liquid comes in contact with the eyes. Skin contact: may cause skin irritation. Repeated and prolonged contact with skin may cause allergic dermatitis. Inhalation hazards: exposure to high concentrations of vapors may cause dizziness, breathing difficulty, headaches or respiratory irritation. Extremely high concentrations may cause drowsiness, staggering, confusion, unconsciousness, coma or death. Liquid or vapor may be irritating to skin, eyes, throat, or lungs. Intentional misuse by deliberately concentrating and inhaling the contents of this product can be harmful or fatal. Ingestion hazards: moderately toxic. May cause stomach discomfort, nausea, vomiting, diarrhea, and narcosis. Aspiration of material into lungs if swallowed or if vomiting occurs can cause chemical pneumonitis which can be fatal. Target organ: chronic overexposure to a component or components in this material has been found to cause the following effects in laboratory animals:
-
Kidney damage Chronic overexposure to a component or components in this product has been suggested as a cause of the following effects in humans:
-
Cardiac abnormalities
|